

|
A wavelength is one cycle of a wave, and we measure it as the distance between any two consecutive peaks of a wave. The peak is the highest point of the wave, and the trough is the lowest point of the wave. |
So, if the wavelength of a light wave is shorter, that means that the frequency will be higher because one cycle can pass in a shorter amount of time. This means that more cycles can pass by the set point in 1 second. Likewise, a light wave that has a longer wavelength will have a lower frequency because each cycle takes a longer time to complete.
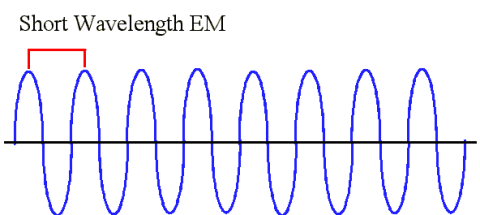
|
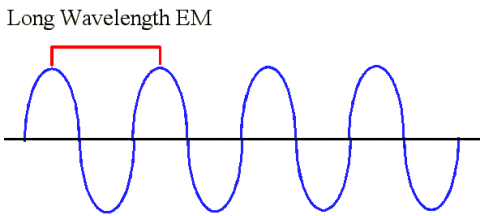
|
| This is an example of a shorter wavelength. Like we said earlier, if you were an observer standing on the black line, and you were so quick that you could count the number of wave peaks that passed you in one second, you would find that the number of wave peaks that you counted would be higher than a longer wavelength. |
This is an example of a longer wavelength. Suppose you put yourself on the line again and you start clocking the
number of wave peaks that pass you. Wait a minute, not nearly so many wave peaks have gone by in one second like
before with the shorter wavelength. That means that longer wavelengths have a lower frequency.
|
Conclusion: a longer wavelength means a lower frequency, and a shorter wavelength means a higher frequency!
To go back to the electromagnetic spectrum.